Can you imagine what relationship there may be between yeast and the new recombinant vaccine against RHD?
Rarely is it possible to have such a headline, but the fact is that both yeast and the new recombinant rabbit haemorrhagic disease vaccine are in some way connected. To understand how, we must first examine how different types of vaccine are produced. The most commonly-used in veterinary medicine are:
- Live attenuated vaccines: effective live attenuated vaccines require limited replication of the vaccine antigen in the animal. With this type of vaccine, it is the individual itself that generates immunity to the pathogen.
- Inactivated vaccines: in these vaccines, the vaccine antigen does not replicate in the animal, as it is inactivated.
Vaccines using recombinant technology, such as classical vaccines, can also be live or inactivated. Recombinant vaccines, developed more recently and using more advanced technology, usually present a part of the pathogen, which confers the best immunity, to the target animal's immune system.
This is how YURVAC® RHD, the new vaccine against rabbit haemorrhagic disease, was developed.
But what is the link between yeast and the vaccine?
In this case, a yeast is used as a technological platform, which integrates the coding of the most immunogenic RHD viral protein, VP-60, into its DNA. This recombinant yeast is cultured in-vitro to produce the vaccine antigen.
Therefore, YURVAC® RHD is a recombinant vaccine in which the active ingredient is the recombinant capsid protein of the RHDV2 virus, which is in the form of VLPs (virus-like particles). But what are VLPs?
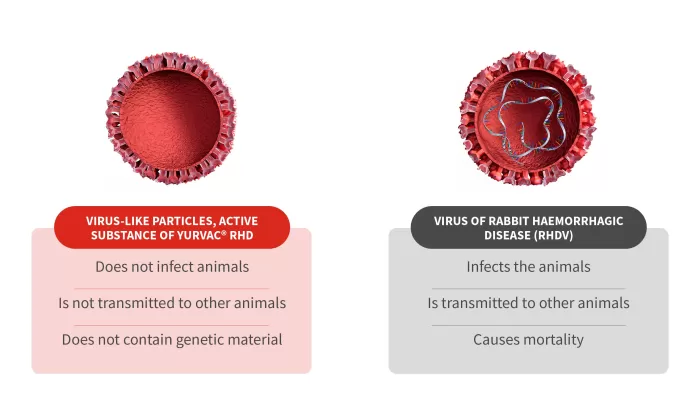
VLPs are molecules that display viral surface proteins in an appropriate configuration, which elicits a strong immune response. They are usually derived from viral envelope proteins and are not contagious as they lack genetic material and therefore cannot replicate.
Each recombinant yeast produces a multitude of VP-60 protein monomers in the manufacturing process, and when all these monomers are released, they self-assemble into VLPs, mimicking the outer capsid of the RHD virus.
YURVAC® RHD is also an O/W (oil/water) emulsion that interacts with mononuclear cells, such as phagocytic cells or antigen-presenting cells. It also promotes the gradual and continuous release of the antigen.
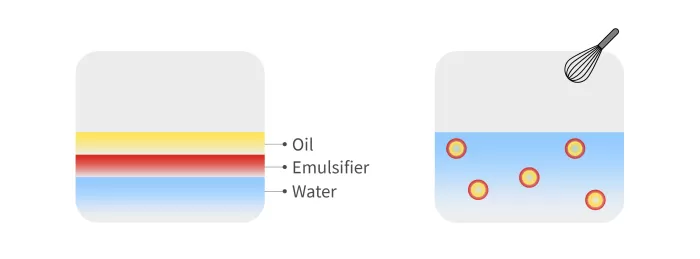
Figure 1: Diagram of how the O/W emulsion is produced.
Figure 1 shows schematically how this type of emulsion is produced, which can be thought of in simple terms as like making mayonnaise.
However, in this case, the proportion of each of the components is precisely specified. An emulsifier is also added to coat each of the oil droplets to keep them stable. Droplet size and emulsion stability are also key factors in the vaccine.
YURVAC® RHD is indicated for the active immunisation of rabbits from 30 days of age onwards to reduce mortality of rabbit haemorrhagic disease (RHD) caused by both the classical RHD virus (RHDV) and variant strains (RHDV2), including highly virulent strains. A single annual dose will therefore protect rabbits against rabbit haemorrhagic disease without the need to discriminate between classical and variant strains.
What is meant by highly virulent strains?
In recent years, an increase in virulence has been detected in RHDV2 strains compared to the virulence of the initial isolates. This means that the degree of pathogenicity is higher in some of the currently isolated strains, resulting in higher mortality. Therefore, one of the main challenges that YURVAC® RHD had to overcome was to be able to protect against these types of strain, which was demonstrated by conducting efficacy studies 7 days post-vaccination and 1 year post-vaccination.
Furthermore, efficacy tests were not only carried out with a highly virulent strain, but also with the classical strain. The results also showed that YURVAC® RHD is able to protect against this virus (RHDV). This means that a single annual dose (0.5 ml/rabbit) administered subcutaneously protects rabbits against the classical strain (RHDV) and the variant strain (RHDV2), including highly virulent strains.
Vaccination of young rabbits
One of the main differences between the classical strain (RHDV) and the variant strain (RHDV2) is that variant strains can also affect young rabbits. Early vaccination will help to reduce cases of rabbit haemorrhagic disease. YURVAC® RHD can be administered from 30 days of age without affecting maternal immunity.
Safety tests have also been carried out to verify its use in companion animals. The reproductive indices of vaccinated rabbits during gestation and lactation were also examined. The results showed that YURVAC® RHD can be used in both scenarios.
So what is the link between yeast and the vaccine?
YURVAC® RHD is the first commercial veterinary vaccine that uses yeast as a technology platform to produce VLPs.
References
WOAH Terrestial Manual 2023. Chapter 3.7.2. – Rabbit haemorrhagic disease.
YURVAC® RHD Recombinant vaccine against rabbit haemorrhagic disease, in injectable emulsion.
COMPOSITION: Each dose of 0.5 ml contains: recombinant RHDV2 virus capsid protein, RP*≥ 0.7, (*) Relative Potency (ELISA test). Adjuvant: Light mineral oil. INDICATIONS: Rabbits: For active immunisation of rabbits from 30 days of age onwards to reduce mortality of rabbit haemorrhagic disease (RHD) caused by classical RHD virus (RHDV) and variant strains (RHDV2), including highly virulent strains. ADMINISTRATION ROUTE: Subcutanous use. DOSAGE: 0.5 ml/animal. Primary vaccination: rabbits from 30 days of age onwards. Revaccination: annually. ADVERSE REACTIONS: Very common: elevated temperature, the highest individual rectal temperature increase was 1.15 ºC which returned to normal values 24 hours later. Very common: injection site inflammation (< 2 cm) can be observed. These local reactions gradually reduce and disappear without need for treatment. WITHDRAWAL TIME: 0 days. SPECIAL PRECAUTION: Vaccinate healthy animals only. Do not use in cases of hypersensitivity to the active substance, to the adjuvant or to any of the excipients. Pregnant does should be handled gently to avoid stress and risk of abortion. No safety study on the reproductive performance has been conducted in male rabbits (bucks). Special precautions to be taken by the person administering the veterinary medicinal product to animals: To the user: This veterinary medicinal product contains mineral oil. Accidental injection/self-injection may result in severe pain and swelling, particularly if injected into a joint or finger, and in rare cases could result in the loss of the affected finger if prompt medical attention is not given. If you are accidentally injected with this veterinary medicinal product, seek prompt medical advice even if only a very small amount is injected and take the package leaflet with you. If pain persists for more than 12 hours after medical examination, seek medical advice again. To the physician: This veterinary medicinal product contains mineral oil. Even if small amounts have been injected, accidental injection with this veterinary medicinal product can cause intense swelling, which may, for example, result in ischemic necrosis and even the loss of a digit. Expert, PROMPT, surgical attention is required and may necessitate early incision and irrigation of the injected area, especially where there is involvement of finger pulp or tendon. Allow the vaccine to reach room temperature before use. Shake well before administration. No information is available on the safety and efficacy of this vaccine when used with any other veterinary medicinal product. A decision to use this vaccine before or after any other veterinary medicinal product therefore needs to be made on a case by case basis. Do not mix with any other veterinary medicinal product. Store and transport refrigerated (2 °C – 8 °C). Do not freeze. Keep the vial in the outer carton in order to protect from light. FURTHER INFORMATION: Onset of immunity: 7 days for RHDV2; 14 days for RHDV. Duration of immunity: 1 year. Can be used during pregnancy and lactation. The vaccine is intended to stimulate active immunity against RHDV and RHDV2. The active substance of the vaccine is the recombinant RHDV2 capsid protein, which auto-assembles into Virus Like Particles (VLPs). PACKAGING: 10 glass vials of 1 dose (0.5 ml); 1 glass vial of 10 doses (5 ml); 1 PET vial of 40 doses (20 ml); 1 PET vial of 200 doses (100 ml). MARKETING AUTHORISATION NUMBER(S): EU/2/23/298/001; EU/2/23/298/002; EU/2/23/298/003; EU/2/23/298/004. NAME OF THE MARKETING AUTHORISATION HOLDER: LABORATORIOS HIPRA, S.A. Veterinary medicinal product subject to prescription.
